Munguva pfupi yadarika, StrongStep® SARS-CoV-2 Antigen Rapid Test (Professional Edition) kubva kuNanjing Liming Bio-products Co., Ltd. yakawana Singapore HSA certification, Malaysia (MDA) yakakurudzirwa runyorwa, uye iri kuUK Dhipatimendi reHutano uye. Human Services (DHSC) yakaongorora yakazvimiririra uye yakagamuchira rumbidzo.
Izvi zvisati zvaitika, StrongStep® SARS-CoV-2 antigen yekuona kit kubva kuNanjing Liming Bio-products Co., Ltd. yakawana zvakatevedzana EU CE certification, China National Institutes for Food and Drug Control (NIFDC) yekunyoresa yekuongorora yekuongorora, yakapinda muRockefeller. Nheyo yakakurudzira runyorwa, chitupa cheGerman Federal Agency for Medicines and Medical Devices (BfArM) certification, , Guatemala certification, Indonesian FDA certification, Italian Ministry of Health certification, Philippines FDA certification, Singapore HSA certification, Ecuador certification, Brazil (ANVISA) chitupa, Chile chitupa. , Argentina certification, Dominica certification, Guatemala certification uye zvimwe zvitupa.Parizvino, South Africa, India, WHO's EUL, FDA's EUA, European whitelist uye zvimwe zvitupa zvitupa zviri kuitika.
Mufananidzo tsime: Yakakurudzirwa neMalaysia Ministry yehutano

Mufananidzo: Singapore HSA certification
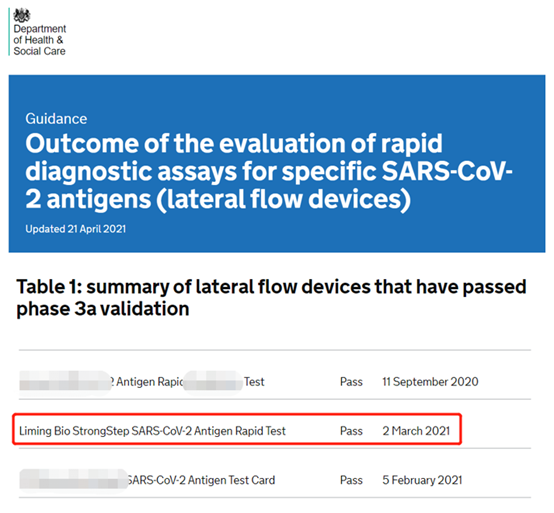
(Mufananidzo sosi: DHSC yepamutemo webhusaiti yeBritish Dhipatimendi reHutano neHuman Services)
Muna 2020, Dhipatimendi rehutano neHuman Services kuUnited Kingdom richanyatso simbisa mareji ekuongorora eCCIDID-19 achipinda munyika kuti ave nechokwadi chekuti akakwana uye akavimbika.Pane zvigadzirwa zana nemakumi maviri zviri kutora chikamu muhurongwa hwekusimbisa, izvo chete zvigadzirwa gumi nepfumbamwe zvakapfuura kuongororwa.Mushure memwedzi mitanhatu yekuomesesa kudzokororwa nekusimbisa, mazana maviri emhando dzemhando uye zviuru zvisina kunaka zvakaratidza zvizere kuita kwepamusoro kweNanjing Liming Bio-products Co., Ltd yekukurumidza kuona reagent yeCOVID-1,000.
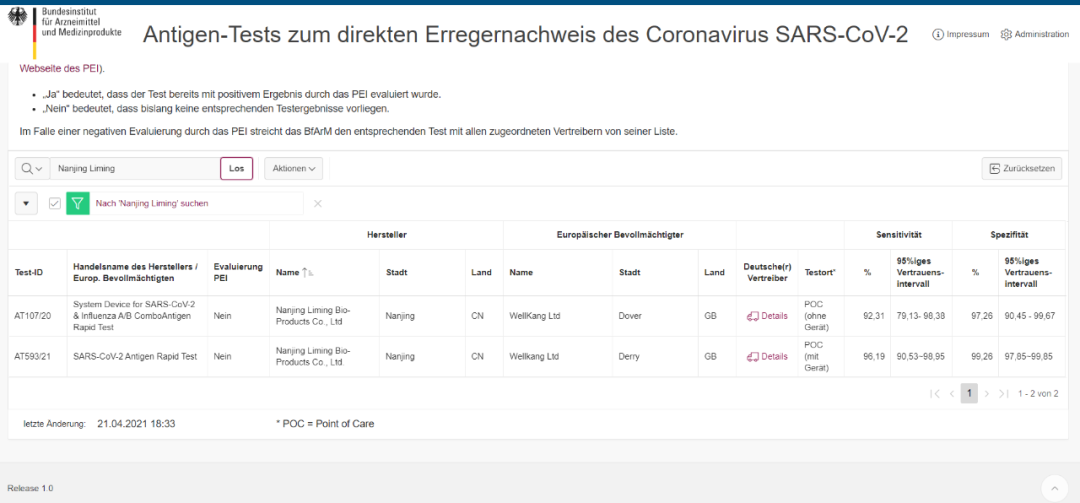
(Mufananidzo unotangira: Webhusaiti yeGerman Federal Agency yeMishonga uye Medical Devices (BfArM))
Official certification Test-ID: AT593/21
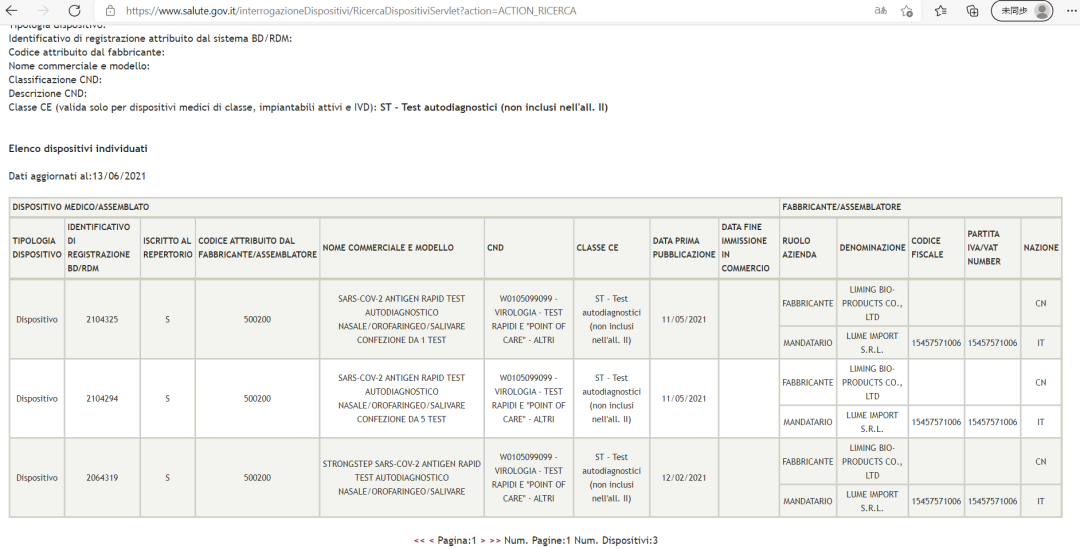
StrongStep® SARS-CoV-2 Antigen Rapid Test self-test version (Self-testing chikamu) yakatenderwa neItaly Ministry of Health.
Kwakabva: Webhusaiti yepamutemo yeItaly Ministry of Health (Ministero della Salute)

StrongStep® SARS-CoV-2 Antigen Rapid Test yakarumbidzwa uye yakakurudzirwa nevashandisi veItaly
Iyo SARS-CoV-2 antigen bvunzo inokurumidza, yakarurama, iri nyore kushandisa, uye inoda midziyo yakaderera uye vashandi.Inonyatsokodzera kukurumidza kuferefetwa kwenyaya dzinofungidzirwa dzehombe huru yekorona hutachiona hwehutachiona, kunyanya kukurumidza kuongororwa kweiyo concentrated kuputika.Inogona kushandiswa senzira yekutanga yekudzivirira yekudzivirira denda, inoshandiswa pakuonekwa kwehutachiona hwepakutanga, kubatsira kudzivirira nekudzivirira denda, nekudzora kupararira kwehutachiona.
COVID-19 ichave iri mudenda renguva refu mune ramangwana, uye kudiwa kwekuyedzwa kuchawedzera zvakanyanya.Kune akasiyana maficha ekushandisa, Nanjing Liming Bio-zvigadzirwa Co., Ltd. yakagadzira akasiyana eSARS-CoV-2 yekuona reagents, "SARS-CoV-2 nucleic acid yekuona + SARS-CoV-2 antigen yekuona + SARS-CoV- 2 antibody kuonekwa + SARS-CoV-2 / A uye B antigen katatu kukurumidza bvunzo + SARS-CoV-2 / A uye B nucleic acid katatu bvunzo + SARS-CoV-2 mhuri kuzviongorora "Iyo yakazara-scenario mhinduro inosangana nezvinodiwa yekuona nekudzivirira pamatanho ese pamusika wepasi rose.Batsira zvakakwana kudzivirira uye kutonga kwepasi rose COVID-19 denda uye kudzivirira uye kudzora kwezvirwere zvekufema sefuruwenza.
Nguva yekutumira: Jun-23-2021







