Nguva pfupi yadarika, Nanjing Lomingbio's Lomingbio's Lomingbio's Coronavirus (COV-COV-2) Antigenice Regent Reagent "COV Certified neGerman Federal Agency yemishonga uye zvekurapa mishonga yekutonga (bfarm). Limingbio yave imwe yevashoma vagadziri muChina yakawana cual certification ye bfarm + pei kuGermany. Kurambidza kweBio's Antigen nekukurumidza bvunzo yapfuura yapfuura chitupa chechokwadi chehushumiro hwehutano hwenyika zhinji, izvo zvinonyatsoratidza kuita kit.

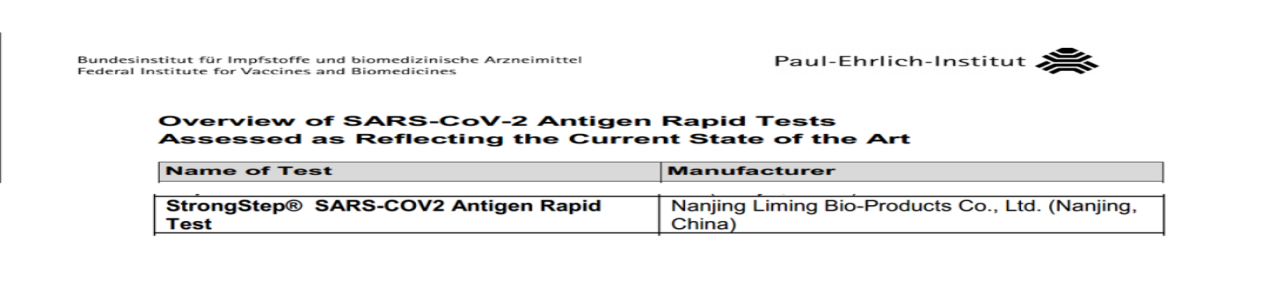
Kurambidza Bio's Antigen Philid Test Test Yakabudirira Kupfuura German Pei Pei Performance Verformation
PS PEI: Paul EGHHLIC Institute (Jeremia: Paul-EFRLICE FEDERAL SEATHINE YEMAHARA YEMABHUKU REMAHARA, Parizvino Ministry Ministry of Health (BMG ), ine mashandiro akazvimirira ekuongorora kwezvigadzirwa zvechigadzirwa, kubvumidzwa kwekiriniki, kubvumidzwa kwechigadzirwa uye kushambadzira, uye batch kuburitsa. Panguva imwecheteyo, iyo zvakare inoitawo kuronga, kudzokororwa kwemirairo yakakodzera, uyeipaSZano resainzi kumasangano akasiyana, kunyanya dzimwe nyika dzeEuropean, European Union neMisimba International. Also, iyoipaSzano rehunyanzvi kuhurumende yeGerman, masangano emuno neParamende, uye anopaSruzivo rwakakodzera kune varwere uye vatengi.
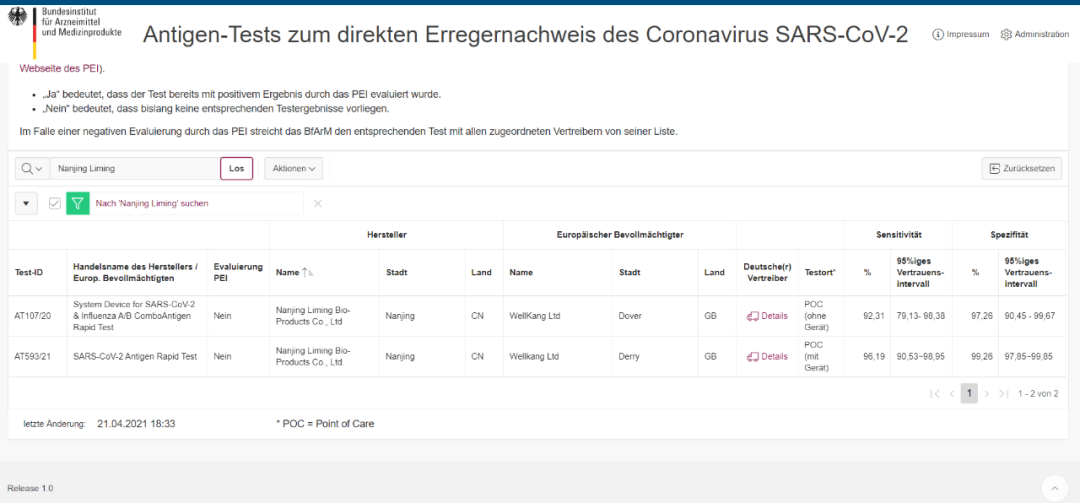
Kurambidza Bio's Antigen Philid Test Test yakabudirira kupfuudza iyo yeGramer Bfm certification
Iyo yakasimba ® inochengetedza-cov-2 antigen nekukurumidza bvunzo yakagadziriswa neEuropean Union (NiFDC) yekunyoresa kuongorora, Certification, Italian Ministry of Health Certification, Certification Certification, Guatemal Certification, Guatemala Certification, Singaphia Certification, Singaphifines chitupa. Yakunda rumbidzo mumucherechedzo wakazvimiririra weBritish Dhipatimendi rehutano uye Services (DHSC) uye (British AAA Certification).

Malawi
Zvimiro uye Zvakanakira
01 Zvakakodzera Sampling: Isiri-Invasive Sampull Counction, Saliva kana Nasophallyaal Swab.
02 Kukurumidza kuona: Iyo yese yekuona maitiro chete inotora maminitsi gumi nemashanu, uye mhedzisiro yacho inoonekwa zvakananga nemaziso.
03 Kureva nyore: Inogona kushanda pasina chero zvigadzirwa zvebetsero uye pasina ruzivo.
04 Zvinonakidza Kuita: Rondedzero ndeye 99.26%, Sensitivity ndeye 96.2%, uye kunyatsojeka ndeye 95%.
05 Kuda Kugadziriswa: Parizvino, kambani ine nyanzvi yekurapa shanduro, yekuzviedza (saliva + Nasophallyaal Swab) vhezheni
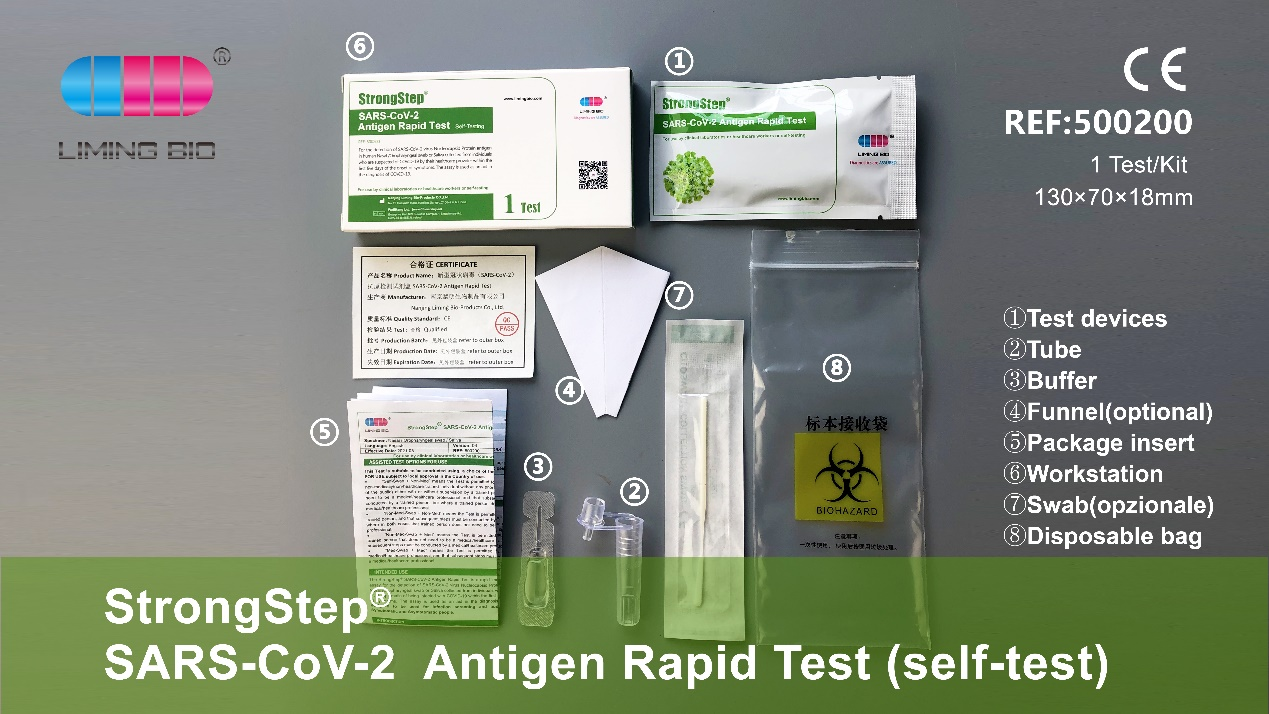
Iyi system mudziyo weSyar-COV-2 Antigen Philid Muedzo (iyo Peni Rudiki Yekuchengetedza Chivako Chiverengero Mufungidziro Kugadzirisa Kugadzirisa Kugadzirisa Kugadzirisa Kugadzirisa Solution yekugadzirisa Operator panguva yekuonekwa kweiyo sars-cov-2 antigen nekukurumidza bvunzo.
Mamiriro epasirese epasi rose mamiriro achiri akanyanya. Nekubuda uye nekupararira kwevaviri vevatirori hutachiona hwehutachiona hwehutachiona hwehutachiona hwehutachiona hwehutachiona hwehutachiona hwehutachiona hwehutachiona hwehutachiona hwehutongi hwakawanda, mamiriro ezvinhu enyika uye matunhu mazhinji akawedzera, uye kudzivirira uye kudzora kuedza kuri kutarisana nematambudziko akakura. Iyo Sars-COV-2 Antigen nekukurumidza bvunzo ndeyekukurumidza, chokwadi, chiri nyore kushanda, uye zvinoda zvishandiso zvakaderera uye vashandi. Zvakakodzera kwazvo kuongorora nekukurumidza kwezviitiko zvekufungidzira nezve rakakura-korona hutsva hwehutachiona hutachiona hwehutachiona hwehutachiona, uye chinonyanya kushanda kuti uwane kuongororwa kwakasarudzika kwekubuda. Inogona kushandiswa seyekutanga mutsara wekudzivirira kwedenda rekudzivirira, zvinoshandiswa pakuonekwa kwekutarisirwa kwekutanga, kubatsira kudzivirira kwehutachiona uye kudzora, uye kudzora kupararira kwehutachiona.
Nanjing Kuramba Bio-Zvigadzirwa C., Ltd. Yakavambwa muna 2001 Iyo ine makore makumi maviri emhando yepamusoro uye yakaunganidza iyo yakazara mhando system, uye yawana iyo is013485 chitupa. Kugadzira manejimendi kunomhanya zvakanyatsoenderana neInternational Earth Management System, inovimbisa kuti zvigadzirwa zvemhando yepamusoro zvinoshanda vatengi pasi rese. It has gradually developed into an internationally renowned large and medium-sized high-tech enterprise specializing in R&D, production, sales and service of in vitro rapid diagnostic reagents.
Kutumira Nguva: Oct-28-2021







